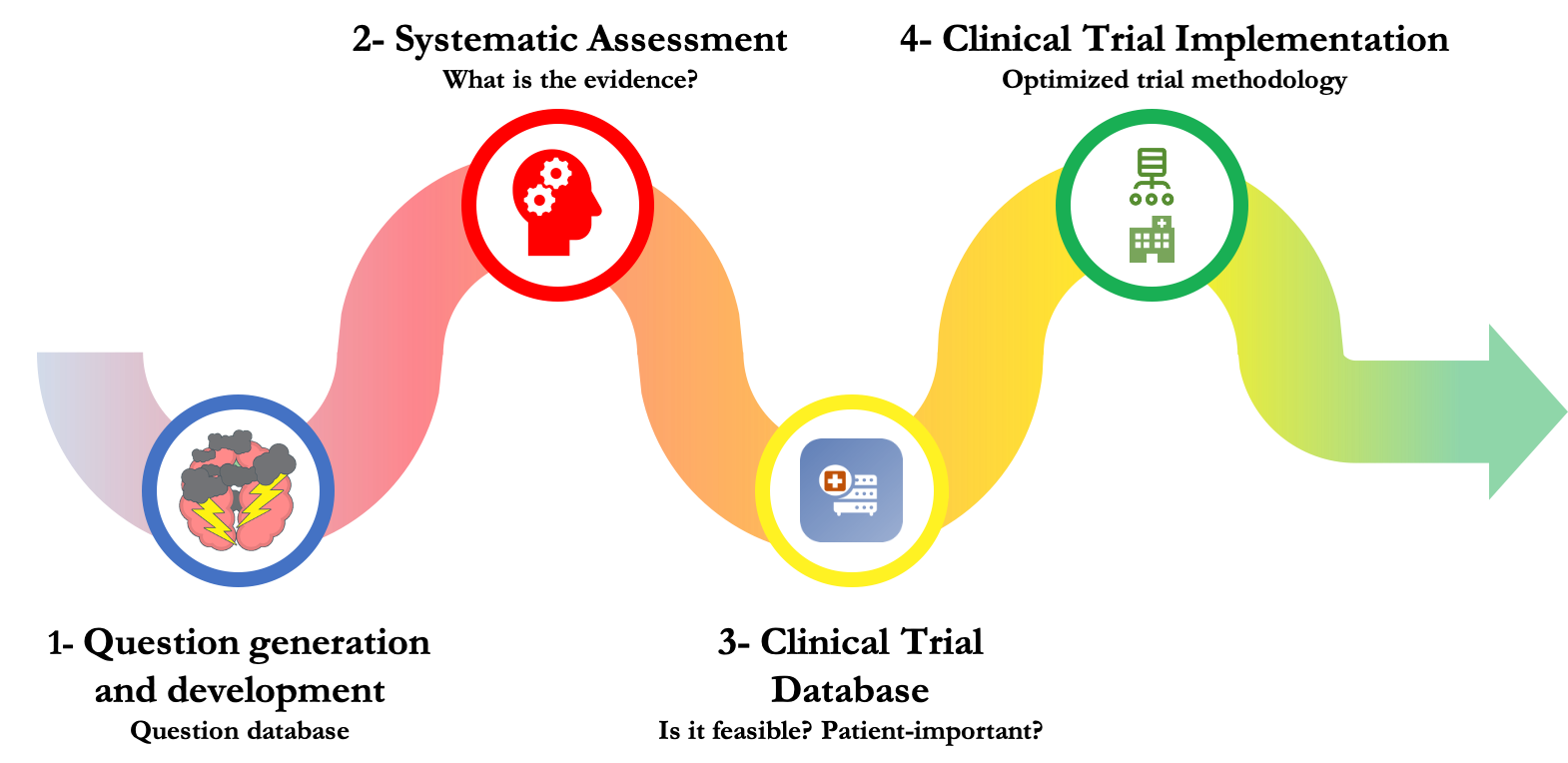

1. Question Generation and Development
We employed several strategies (e.g., in-person networking, conferences, clinical resident teaching opportunities and social media) to identify and compile >200 questions about uncertainty in surgical and perioperative care. Each question was screened for applicability and feasibility, with a focus on short-term outcomes.
Please consider submitting a question! We welcome your collaboration for each submission.
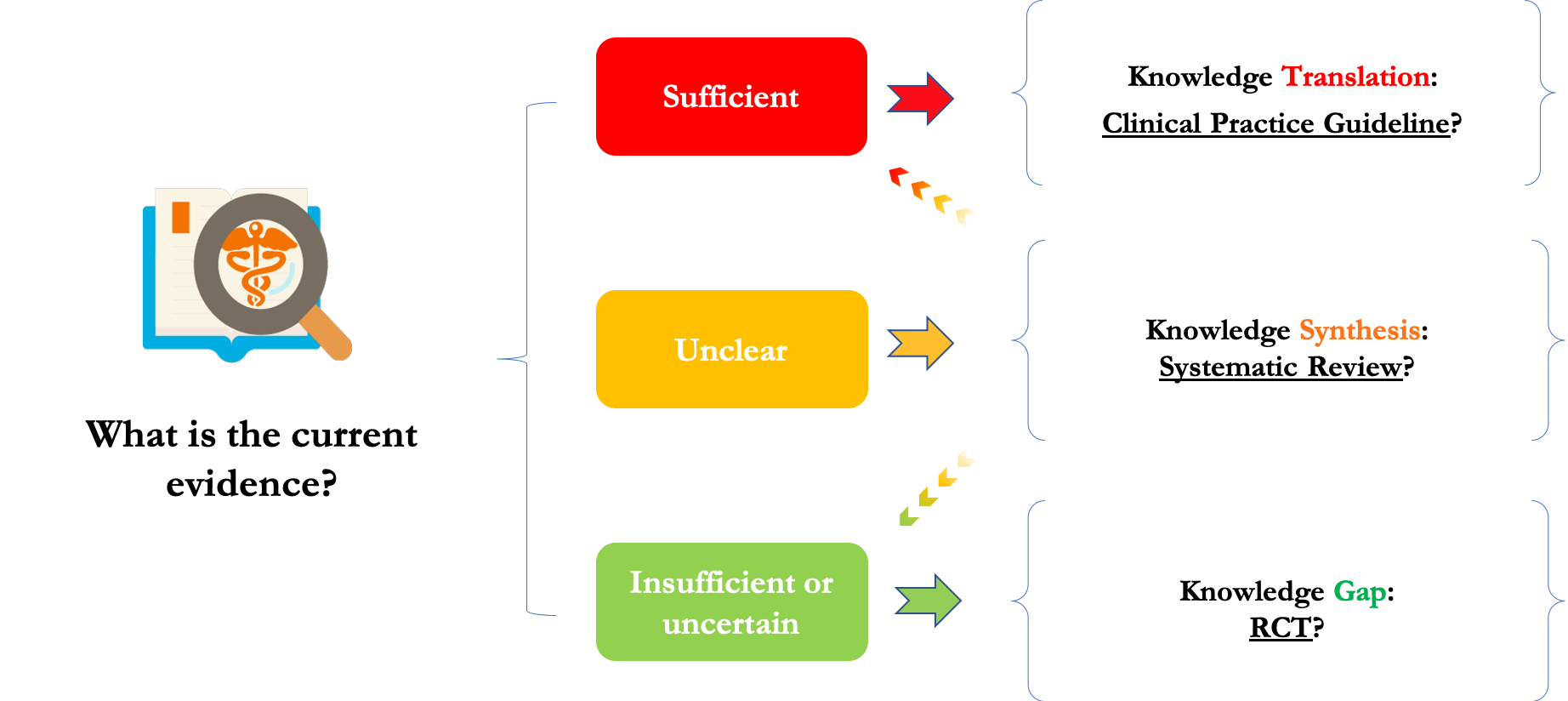
2. Systematic Assessment
We conducted an evidence appraisal of each relevant question using a systematic approach developed by the IMPACTS team. This involved searching and reviewing the recent evidence and categorizing each question as an opportunity for knowledge translation, knowledge synthesis or further clinical investigation (i.e., a knowledge gap).
3. Clinical Trial Selection
We held two stakeholder workshops with healthcare professionals, patient partners, and policy-makers to evaluate and discuss a shortlist of questions (knowledge gaps) according to their impact, feasibility, and applicability. Feedback from these sessions informed the selection of the IMPACTS pilot trials.
4. Clinical Trial Implementation
IMPACTS trials are pragmatic and incorporate several novel design elements to maximize feasibility and efficiency. These include: use of an integrated consent model, web-based enrollment/randomization, an adaptive design, leveraging of existing data collection registries, and an expedited research ethics approval process with REBs and Clinical Trials Ontario.